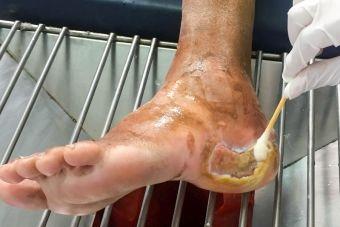
Understanding the Wound Infection Continuum
December 31, 2018
Wound infection is a complex process that can be affected by a variety of factors, some of which inhibit the ability to heal. The first stage of healing, the inflammatory stage, is particularly susceptible to chronicity. Chronicity can be influenced by many factors, with a common contributor being the presence of infection.1
The wound infection continuum begins with contamination and, if left unchecked, will progress to systematic infection. Patients’ medical conditions can influence the likelihood of developing infection for a given wound, but there are also other signs that a wound is experiencing colonization or infection. Because no single test exists that can diagnose infection, it is generally up to the clinician to recognize the signs during assessment. If infection is suspected, DNA swabbing and analysis can provide a greater level of detail about the patient’s microbiome and can often assist with identification of pathogenic bacterial and fungal microorganisms.2 This can also help to identify the best treatment method available. Wound infection development depends upon a variety of microbial and host factors, both of which can change status during any stage of the continuum.
Wound Infection Stages
Contamination
Initial wound contamination is nearly impossible to detect. Because biofilms are present up to 60% of the time, infection is most frequently caused by bacterial colonization originating from either normal flora or bacteria on one’s body.3 When possible, prevention of infection is the optimal goal. Prevention is best achieved by wound cleansing, using a non-cytotoxic wound cleanser or normal saline to reduce debris. This provides the optimal environment for healing.4
Colonization
The differentiation between colonization and infection is challenging to decipher. However, colonization is generally defined as the presence of proliferating or replicating bacteria with no host response.5 Proliferation does not reach a critical level and there are no evident symptoms, such as inflammation. However, gauging the host response is still challenging.
Critical Colonization
Critical colonization is a relatively new concept added to the wound infection continuum in recent decades and is used to describe the condition in which there is “multiplication of organisms without invasion but interfering with wound healing.”6 In this state, wounds often stagnate, rather than improve in condition, and obvious signs of infection such as fever and inflammation tend to be absent. Despite this, other signs, such as the presence of discoloration and odor, may be observed. The bioburden is elevated beyond colonization to a point at which it affects the healing process. The critical colonization stage is also the tipping point on the wound infection continuum where treatment becomes necessary as a means to stop the progression to infection.7 Effective treatment for critical colonization can generally be achieved through the use of topical antiseptics that control the bioburden so that healing can proceed.8 A bioburden level of >105 bacteria per gram of tissue is the threshold at which critical colonization crosses into infection. At >106 bacteria per gram of tissue, healing becomes impeded.9
Infection
Like critical colonization, infection is the invasion of proliferating bacteria that is present not only on the surface of the wound but also in healthy tissue on the periphery of the wound. Infection will cause a host response, but this immunological response will not be sufficient to overcome the bacteria alone.9 During active infection, the wound condition becomes degenerative, as opposed to its stagnation during critical colonization. Signs of inflammation, odor, and discoloration will typically be observed, and bleeding becomes more common.7 Infection will require antibiotic treatment, either topically or orally.
Systemic Antibiotic Versus Topical Antimicrobial Treatment
Antibiotic resistance is becoming an increasingly common problem, and antibiotic regimens should be approached cautiously because it is often possible to treat wounds in the earlier stages of the infection continuum more effectively with another type of treatment. Wound infection treatment must be addressed by the level of bacteria present in the wound. When wounds have a high bioburden, topical antibiotics and antiseptics can decrease the bacterial load.10 However, if a wound is clinically infected, a wound culture is the best way to determine the optimal type of antibiotic.9
References
1. Penhallow KA. A review of studies that examine the impact of infection on the normal wound-healing process. J Wound Care. 2005;14(3):123–6.
2. Mouraviewv V, McDonald M. An implementation of next generation sequencing for prevention and diagnosis of urinary tract infection in urology. Can J Urol. 2018;25(3):9349–56.
3. O’Dell ML. Skin and wound infections: an overview. Am Fam Physician. 1998;57(10):2424–32.
4. Sardina D. Is your wound-cleansing practice up to date? Wound Care Advisor. https://woundcareadvisor.com/is-your-wound-cleansing-practice-up-to-dat…. Accessed December 7, 2018.
5. Ovington L. Bacterial toxins and wound healing. Ostomy Wound Manage. 2003;49:8–12.
6. Landis S, Ryan S, Woo KY, Sibbald G. Infections in chronic wounds. In: Krasner DL, ed. Chronic Wound Care: The Essentials—A Clinical Source Book for Healthcare Professionals. Malvern, PA: HMP Communications; 2014:87–130.
7. Eberlein T. Critical colonisation and local infection—current therapy by use of polyhexanide. https://lohmann-rauscher.co.uk/downloads/clinical-evidence/SXP010-T-Ebe…. Burton on Trent, United Kingdom: L&R Medical. Accessed December 7, 2018.
8. White RJ, Cutting KF. Critical colonization—the concept under scrutiny. Ostomy Wound Manage. 2006;52(11):50–6.
9. Hanft JR, Smith B. How to differentiate between infected wounds and colonized wounds. Podiatry Today. 2005;18(7):85–90.
10. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–69.
The views and opinions expressed in this content are solely those of the contributor, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.