TIMERS: Identifying Tissue Types
April 30, 2020
By the WoundSource Editors Wound bed preparation is a well-established concept, and for many years the TIME framework – consisting of addressing Tissue Management, Inflammation and Infection, Moisture Balance, and Edge or Epithelial Advancement – was the standard tool used by clinicians to manage patients’ wounds throughout the wound care cycle. This framework has recently been reassessed, with a recommendation to become TIMERS, by adding two new categories: R for Regeneration and Repair; and S for Social factors, which comprise an overarching theme that includes patient factors that may impact healing.1
Overview of Additions to TIME
There are several notable additions or changes to the new framework. Although the original TIME framework accounted for the contribution of inflammation and infection to wound chronicity, updated research also considers the complex nature of failure to heal and the role of biofilm. The increased understanding of biofilm and of how to address bioburden provides an opportunity to treat wounds by addressing the root cause of infection and inflammation without unnecessary reliance on antibiotics.2
Additionally, the regeneration and repair category was added to increase the focus on wound closure by providing a matrix to support cell infiltration and by stimulating cell activity through use of modalities such as signal molecules or growth factors, oxygen therapy, negative pressure wound therapy, or stem cells.2 However, wounds are likely to respond to treatment only once other risk factors have been assessed and mitigated. These risk factors may include the underlying disorder, infection, biofilm, and patient-related factors. The addition of the social factors category signifies the importance of encompassing the entire framework, taking a holistic approach to assessing and treating the wound, and recognizing the importance of patient engagement. It also denotes the patient’s social factors that may impact the wound’s ability to progress through the healing cycle, such as access to resources, the ability to find transportation to weekly appointments, the ability to take time off from work, and other related factors.3
Identification of Tissue Types
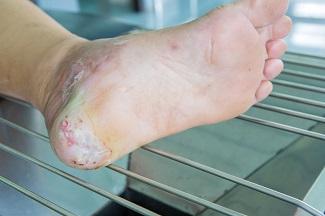
The first category in the updated TIMERS framework remains tissue management, which encompasses the observation of viable or non-viable tissue to identify an appropriate treatment modality. In many instances, removal of non-viable tissue may be achieved through various methods of debridement. The best course of action can be determined only by assessing the comprehensive needs of the patient and the characteristics of the wound itself. However, the recommendations corresponding to the TIMERS framework note that hard-to-heal wounds are unlikely to respond to repair and regeneration therapies unless risk factors have been addressed.1 These risk factors may include the presence of non-viable tissue. This makes it imperative to understand the different types of tissues that may be encountered in the wound bed.
- Necrotic tissue: Necrotic tissue is a result of cell death, which often occurs when noxious stimuli are present, such as bacteria, viruses, fungi, or parasites. It can also be the result of oxygen deprivation or extreme environmental conditions. There are two primary types of necrosis. Necrosis is liquefactive (colliquative) when there is partial or complete dissolution of dead tissue and transformation into a liquid, viscous mass. Coagulative necrosis maintains the typical architecture of the tissue for several days after cell death. In addition to these two primary classifications, there are other types of necrosis (caseous, fat, gangrenous, fibrinoid) that describe the clinical scenarios or organ damage contributing to the necrosis. Antibiotics and surgical management, including drainage of abscesses, wound debridement, and amputation, are standard treatments when necrotic tissue is present.4
- Viable tissue: Viable tissue often appears light pink to red and may be moist. The line between viable tissue and non-viable tissue in a wound is often marked by visible signs of infection or maceration. The E in TIMERS explores factors that may impact edge advancement of the wound. These factors can be extrinsic, such as repeated trauma and poor metabolic control, or intrinsic, such as deficient growth factors or reduced fibroblast activity.5 Epibole formation should also be noted because it can designate a wound as chronic.6
- Granulation tissue: Granulation tissue provides a moist wound environment with active healing. It often appears red, uneven, and dotted. Friable granulation tissue, or tissue that bleeds easily, may indicate the presence of infection.7
- Eschar or slough: Tissue may be yellow, gray, purple, black, or brown and have a soft, slimy consistency, or it can form a hard, leathery eschar. Debridement and moisture management may address this type of tissue, although dry, stable eschar should generally be left in place.7
- Epithelial tissue: This tissue is pale pink with white dots within the wound bed or at the wound edges. Epithelial tissue signifies a healing wound.7
- Tendon, ligament, and bone: Tendon or ligament tissue appears yellow or off white and is shiny unless dehydrated. Bone is white and hard unless it is necrotic. Exposed tendon and ligament can resemble yellow slough, and this often requires the clinician to have a comprehensive understanding of the local anatomy.7
Conclusion
Clinicians should take care to become familiar with the different tissue types that may be present in a wound bed, to devise a comprehensive plan of care. Care plans for hard-to-heal wounds will consider how to manage wound symptoms, as well as factors that will contribute to closing the wound.
References 1. Atkin L, Bucko Z, Conde Montero E, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;28:3a. 2. Stevenson P, Schultz G. 2019 international consensus includes biofilm treatment as new standard of care. Wound Manag Prev. 2019;65(7). https://www.o-wm.com/article/2019-international-consensus-includes-biof…. Accessed April 16, 2020. 3. Sin PJ. Change in chronic wound management, new TIMERS guideline further improves wound care. MIMS Multidisciplinary. 2019. https://specialty.mims.com/topic/change-in-chronic-wound-management--ne…. Accessed April 16, 2020. 4. Adigun R, Basit H, Murray J. Necrosis, cell (liquefactive, coagulative, caseous, fat, fibrinoid, and gangrenous). StatPearls.com. 2019. https://www.statpearls.com/sp/md/480/24339/. Accessed April 16, 2020. 5. Edmonds M, Foster AVM, Vowden P. Wound bed preparation for diabetic foot ulcers. In: Calne S, ed. Wound Bed Preparation in Practice. European Wound Management Association (EWMA). London: MEP, Ltd.; 2004. 6. Corum L. Ulcer and wound care: getting to the depth of the tissue. National Center of Continuing Education. 2019. https://nursece.com/courses/132-ulcer-and-wound-care-getting-to-the-dep…. Accessed April 16, 2020. 7. Young T. Accurate assessment of different wound tissue types. Wound Essentials. 2015;10(1):51-54.
The views and opinions expressed in this content are solely those of the contributor, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.