Venous Leg Ulcers in Diverse Populations
May 2, 2023
Introduction
Venous Leg Ulcers (VLUs) are among the most widespread chronic lower extremity wounds, with approximately 70% of chronic leg ulcers reported as VLUs.1 Across the population in the western hemisphere, it's estimated that 2% have VLUs, while those in the aging population have a greater prevalence at 5%.2 In the United States, 6 million people struggle with these wounds, a number dwarfed by those in other countries. For instance, in Africa, approximately 25-135 million individuals have VLUs. Several populations face greater occurrence and severity of VLUs, including those aged 65 and over, those with pigmented skin, and the female demographic. Across these populations, different factors put each population at risk. For instance, in hard-to-heal VLUs, researchers have found race to be a novel risk factor, even in circumstances where non-white patients had equal access to care as white patients—suggesting that “other factors” may be the cause of this discrepancy in healing.3
In contrast, for those of the aging population, the greatest risk factors for VLU development and chronicity concern the fragility of skin and the presence of concomitant health conditions, like diabetes.4 For these reasons, wound care professionals must familiarize themselves with the intricacies of care necessary to treat patients with VLUs across diverse populations to reach best outcomes for these vulnerable patients.
How to Treat VLUs Across At-Risk Populations
Overall, best practices for the diagnosis and treatment of VLUs may include the following4,5:
- Clinical examination, color flow duplex, and doppler sonography (ultrasound)
- Compression therapy
- Physical therapy
- Manual lymphatic drainage
- Endovascular procedures (ie, Venous Ablation)
- Phlebotonics
Many of the above assessment and treatment options will require the help of a multidisciplinary team, including vascular specialists, physical therapists, nurses, and wound care professionals.4 Despite compression therapy being a gold standard, clinicians may hesitate to use it in some contexts due to the risk of mixed etiology pathologies. Using proper methods to evaluate for arterial insufficiency can alleviate these concerns. With the help of a vascular specialist, methods such as transcutaneous oxygen pressure (TcPO2), ankle-brachial pressure index (ABPI), and toe pressure can be used to assess for both micro- and macrocirculation, respectively.4 Some experts recommend using compression therapy with another conservative therapy for best outcomes once mixed etiology is ruled out.
How much do you know about Managing Wounds in Diverse Patient Populations? Take our 10-question quiz to find out! Click here.
In contrast, according to a recent consensus document, compression therapy may still be recommended despite the presence of minor arterial insufficiency as long as the pressure applied does not "exceed the local arterial perfusion pressure." 4 However, clinicians should remain cautious when applying compression therapy in patients with arterial inflow, as it may prove dangerous and painful.4 Elastic (long stretch) and inelastic (short stretch) bandages provide varying forms of compression dependent on if the patient is at rest. Elastic bandages provide more pressure at rest, while inelastic bandages compress more during patient activity. Because of its high adherence rates and a better ability to reduce venous reflux, inelastic bandages tend to be more effective. It is important to note that although compression therapy provides best outcomes for patients with VLUs, it does not treat the root cause. Endovascular procedures and certain phlebotonics have been found to reduce wound reoccurrence, edema, and inflammation.4
Female Population
Risk Factors
As many wound care professionals are familiar, VLUs are a result of chronic venous insufficiency (CVI). This condition has a myriad of risk factors, both modifiable and non-modifiable, such as genetics, prolonged standing, age, and trauma, to name a few. Of note, the non-modifiable risk factor of female (assigned at birth) gender and modifiable risk factor of past pregnancy increase likelihood of CVI development.4 Other risk factors include several conditions female patients have an increased risk of developing, including the following:
- Deep vein thrombosis (DVT)
- Post-thrombotic syndrome (PTS)
- Varicose veins
These risk factors help to explain why female patients are at higher risk for VLUs. Pelvic congestion syndrome (PCS) is theorized to also contribute to this discrepancy. PCS can occur during menstruation or pregnancy and is impaired circulation of pelvic veins indicated by pelvic pain.5
Best Practices
Since female patients have a higher risk of edema, it is important to differentiate between the etiology of edema for proper treatment of the underlying cause. Wound care professionals can differentiate between these 2 etiologies by observing whether the foot or ankle/leg has swelling. Typically in lymphedema, the second digit of the foot may exhibit swelling, while edema from CVI typically only appears in the leg or ankle.6 If a patient has persistent edema, the lymphatic system may be damaged, leading to lymphedema, further complicating differential diagnosis. A vascular specialist may use Doppler ultrasonography and color flow duplex ultrasonography to confirm edema caused by CVI.4 Compression therapy may be recommended for pregnant patients to mitigate venous insufficiency symptoms in the lower extremities.7
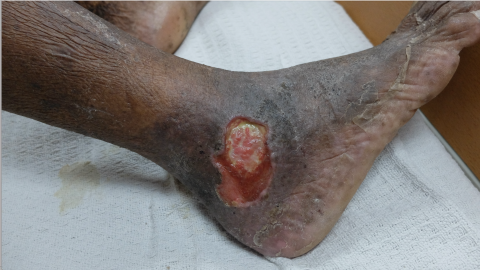
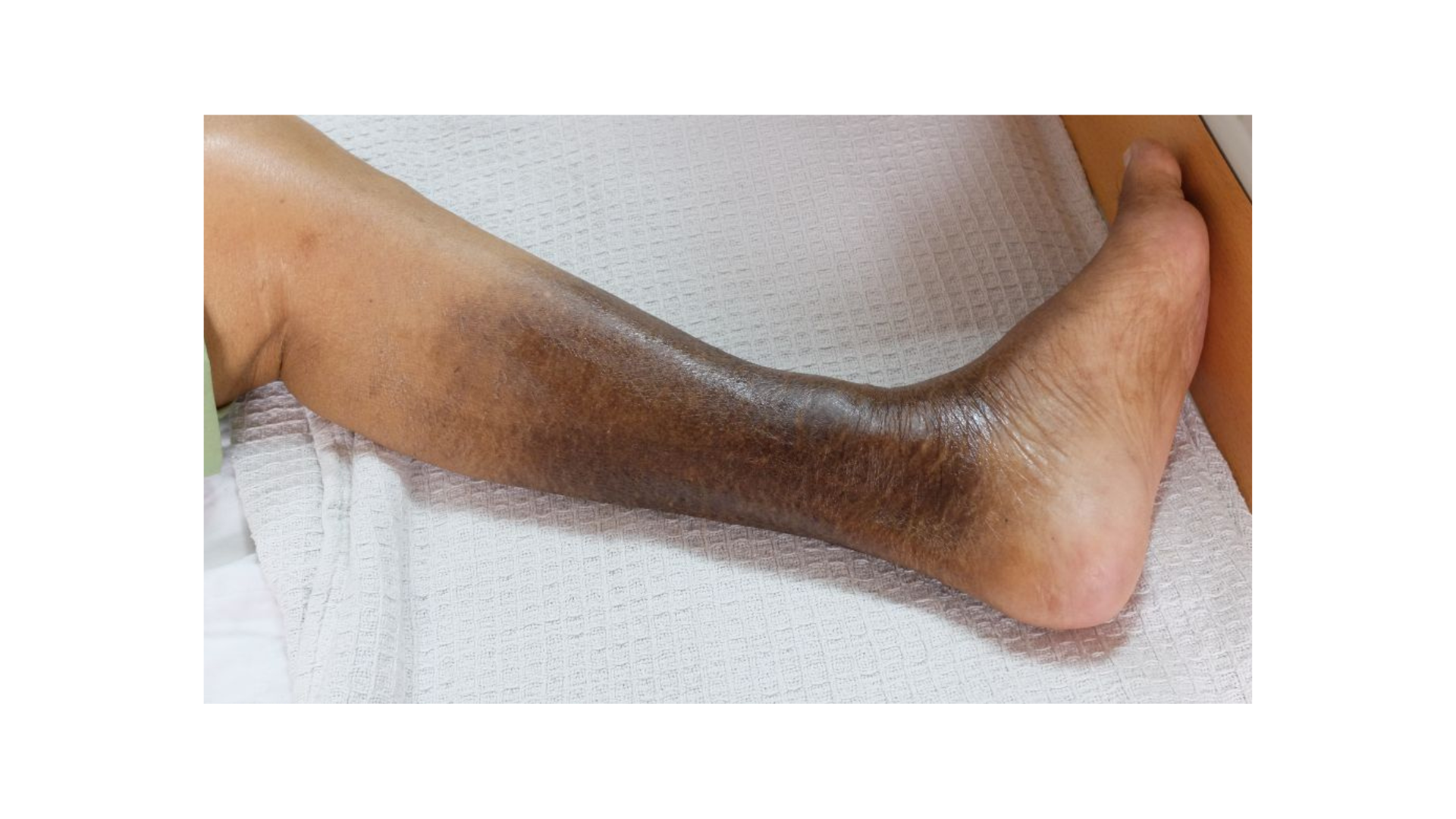
Patients With Skin of Color
Risk Factors
The literature cites essential differences in the presentation of injuries and assessing erythema among varying skin tones.8 However, there is less available data on venous and vascular wounds in this population. Much in the same way that African American patients have been found to present with more significant PIs, these patients also present with more severe chronic venous disease (CVD).9 Experts theorize that lower rates of endovascular abdominal aortic aneurysm (AAA) repair, revascularization, and overall aggressive treatment of venous disease contribute to the higher rates of severe CVD in this population.9 Also of note, there are increased amputation rates in relation to peripheral arterial disease (PAD) among this population, even when revascularization rates were higher.9
Best Practices
Clinicians working with patients with skin of color must ensure they assess for CVI early to intervene procedurally at earlier rates.9 To improve visual assessment of the patient, ensure the area of assessment has appropriate lighting. To determine any discoloration present, compare the area of concern to a portion of the patient's skin that does not exhibit an injury. Besides visual assessment, wound care professionals should use doppler sonography if a patient reports edema, heaviness of the extremities, or nighttime leg cramps.10
Aging Population
Risk Factors
The aging population refers to individuals who are aged 65 and over.6 The prevalence of VLUs in this population is estimated at 1.7% annually, with peak prevalence between 60-80 years of age.6 This patient population struggles with compounding chronic diseases and, consequently, multiple medications.6 They may also experience difficulty or inability to ambulate, social isolation, reduced skin barrier function, and a lower production rate of keratinocytes.11-13
Best Practices
Those in the aging population may have conditions that make them unfit for compression therapy outside of arterial disease. These include but are not limited to the following14:
- Musculoskeletal conditions
- Uncompensated organ failure
- Neuropathy
- Ischemic pain at rest
If a patient from the aging population has any condition that makes them unfit for compression or surgical intervention, clinicians may consider sclerotherapy. For this treatment to create localized thrombosis, the clinician injects a chemical solution into an insufficiently functioning vein, triggering an inflammatory response in the vessel wall's endothelium.15 A 2016 study analyzed the results of this treatment and found that patients in the dataset had lower rates of varicose veins and better quality of life.16
A Note on Adherence
Despite the evidence surrounding the standard of care for VLU treatment, it is often discontinued across care settings. For patients of diverse populations, this discontinued care can needlessly overcomplicate already complex wounds. However, literature continues to show this occurrence. A 2023 study of one UK hospital identified 3 obstacles: clinician education regarding compression therapy, patient adherence, and the presence of equipment and specialized staff.17 In terms of adherence to compression therapy, evidence suggests clinicians understand patients’ specific lifestyles and apply that knowledge to choose a type of compression for the patient. The provision of aids to ensure patients can perform self-care activities like showering was also essential, especially for patients of the aging population. Last, some patients may not be able to tolerate compression due to the pain, and those patients need support and advice from staff.17,18
Conclusion
It is important to note that the economic burden of these VLU wounds in the United States ranges from $15,000 to $34,000 per individual, not including other factors, such as missed workdays.1 Experts report a staggering $14.9 billion is spent annually by taxpayers in the United States on VLUs.2 These statistics, of course, do not include the psychological burden that also affects these patients, such as isolation, depression, lack of sleep, and dependency, to name a few.1 As VLUs continue to burden patients and the health care system, continuous research of solutions and education of best practices is vital. Through best practices and the multidisciplinary team, wound care professionals can help patients across populations who suffer from these wounds.
References
- Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vascular Med. 2022;27(1):63-72. https://journals.sagepub.com/doi/pdf/10.1177/1358863X211028298
- Raffetto J, Ligi D, Maniscalco R, et al. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J Clin Med. 2021; 10(1):29. https://doi.org/10.3390/jcm10010029
- Melikian R, O’Donnel TF, Suarez L, et al. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. Vasc Surg Venus Lymphat Disord. 2019;7(1):98-105. https://doi.org/10.1016/j.jvsv.2018.07.014
- Bernatchez SF, Eysaman-Walker J, Weir D. Venous Leg Ulcers: A Review of Published Assessment and Treatment Algorithms. Adv Wound Care. 2021;11(1):28-41. http://doi.org/10.1089/wound.2020.1381
- Placke JM, Jockenhofer F, Benson S, et al. Venous ulcerations occur more frequently in women on the left lower leg. Can pelvic congestion syndrome be an often undetected cause? Int Wound J. 2020;17(1):230-231. doi: 10.1111/iwj.13260
- Lymphedema. The Vein Center of Arizona. Accessed March 9, 2023. https://www.veincenterofarizona.com/chronic-venous-insufficiency/lymphe….
- Heller J, Canner J, Lum WY, Tsuchiya K. Compression Stockings During Pregnancy: Superfluous? A Pilot Study. J Vasc Surg. 2016;4(1):148. doi:https://doi.org/10.1016/j.jvsv.2015.10.038
- Gunowa N, Hutchinson M, Brooke J, et al. Pressure injuries in people with darker skin tones: A literature review. J Clin Nurs. 2018;27(17-18):3266-3275. doi:10.1111/jocn.14062
- Dua A, Desai S, Heller JA. The Impact of Race on Advanced Chronic Venous Insufficiency. Ann Vasc Surg. 2016;34:152-156.
- Chronic Venous Insufficiency (CVI). Cleveland Clinic. Updated July 17, 2022. Accessed March 22, 2023. https://my.clevelandclinic.org/health/diseases/16872-chronic-venous-ins….
- Pugliese DJ. Infection in Venous Leg Ulcers: Considerations for Optimal Management in the Elderly. Drugs Aging. 2016;33:87-96. https://link.springer.com/article/10.1007/s40266-016-0343-8
- Aguiar ACSA, Sadigursky D, Martins LA, et al. Social Repercussions experienced by elderly with venous ulcer. Rev Gaucha Enferm. 2016;37(6):e55302. https://doi.org/10.1590/1983-1447.2016.03.55302
- Banvolgyi A, Grog A, Gado K, et al. Chronic wounds in the elderly: Decubitus, leg ulcers, and ulcers of rare aetiology. DHS. July 5, 2022;4(4):81-85. https://doi.org/10.1556/2066.2022.00054
- Guideline: Application of Compression Therapy to Manage Venous & Mixed Venous/ Arterial Insufficiency. British Columbia Provincial Nursing Skin and Wound Committee. Published 2016. Updated 2019. Accessed 2023. https://www.clwk.ca/get-resource/compression-therapy-for-venous-insuffi….
- Santler B, Goerge T. Chronic venous insufficiency — review of pathophysiology, diagnosis, and treatment. J German Soc Derm. 2017;15(5):538-556. https://doi.org/10.1111/ddg.13242
- Isobe J, Onyeachom U, Taylor R, Dimitropoulos S. Sclerotherapy Use for Chronic Venous Insufficiency Across the United States: A Report From the Venous Patient Outcome Registry. J Vasc Surg. 2016;4(1):144-145. doi:https://doi.org/10.1016/j.jvsv.2015.10.030
- Lian Y, Birt L, Wright D. Hospital clinicians’ perspectives of using compression on venous leg ulcers: a systematic qualitative review. Br J Nurs. 2023;32(4). https://doi.org/10.12968/bjon.2023.32.4.S30
- Perry C, Atkinson RA, Griffths J, et al. Barriers and facilitators to use of compression therapy by people with venous leg ulcers: A qualitative exploration. J Adv Nurs. 2023. https://doi.org/10.1111/jan.15608
The views and opinions expressed in this blog are solely those of the author, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.









Follow WoundSource
Tweets by WoundSource