A Case Study on the Combined Use of an Epidermal Autograft and NPWT
January 21, 2016
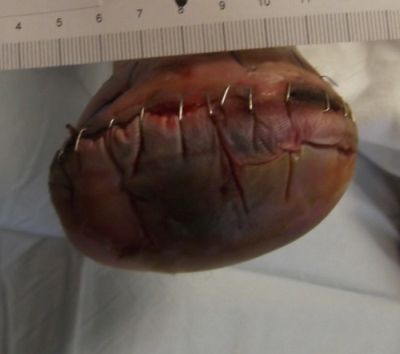
This is the account of a patient case in which technology, clinician experience, and patient adherence converged to save a limb. In August of 2013, a 59 year old female with noninsulin-dependent diabetes mellitus, severe peripheral artery disease, a history of tobacco abuse and a recent right transmetatarsal amputation (TMA) was referred to our hospital-based outpatient department by her home health nurse. Her surgery was one month prior to presentation. During her intake visit with the foot and ankle surgeon, she recounted her post-surgical instructions to include "wash incision once daily with betadine." The home health nurse had referred her back to the surgeon's office with concerns that the surgical incision was not approximating beneath the staples and wound closure strips. At that time, the surgeon removed all closure devices, leaving the wound open. He did not change the patient's care regimen.
Assessment during her first visit to the clinic revealed trophic skin changes of chronic ischemia, and non-palpable dorsal pedis (DP) or posterior tibial (PT) pulses. Capillary refill was sluggish. The contralateral limb presented similarly. The periwound was boggy and mottled, and the wound bed was a dry, dull red. Bone from the first metatarsal was visible, but appeared viable. Both bone and tissue specimens were taken for PCR (polymerase chain reaction) plus culture and sensitivity, of which only the tissue revealed significant growth of pathogens; osteomyelitis was not suspected at this juncture. The patient's wound was covered with a moisture retentive dressing and she was immediately referred to the lower extremity vascular specialist at the neighboring heart institute, where she underwent successful, albeit complex, vascular intervention.
Combining Wound Therapies
Within an 8 week period, she received targeted oral antimicrobial therapy and conservative wound care inclusive of sharp debridement, strict offloading, and dressings to promote moisture and healthy tissue proliferation in the wound bed. The patient-centered plan also included glucose control, dietary modification to include protein supplementation, and smoking cessation. Despite all the above measures, the wound continued to probe to bone, and showed little signs of healing.
9-27-13
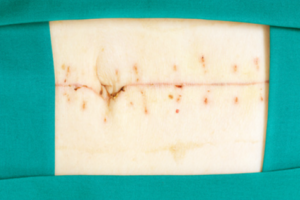
10-7-13
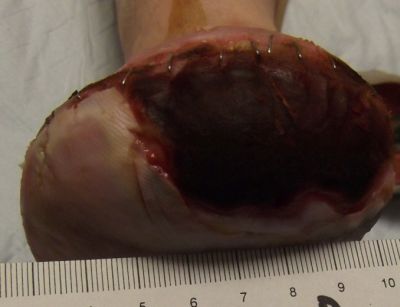
10-23-13
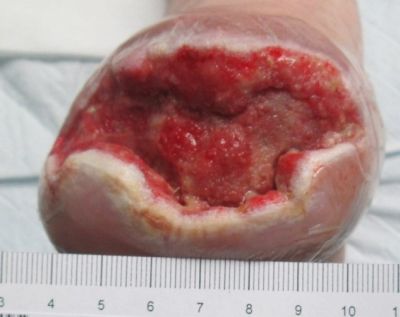
11-4-13
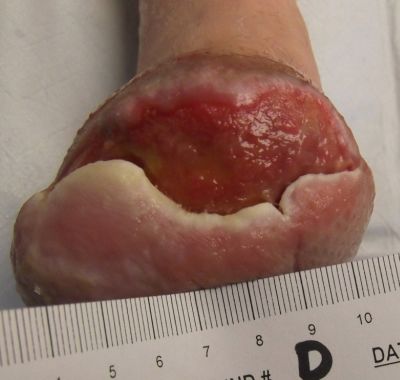
11-25-13
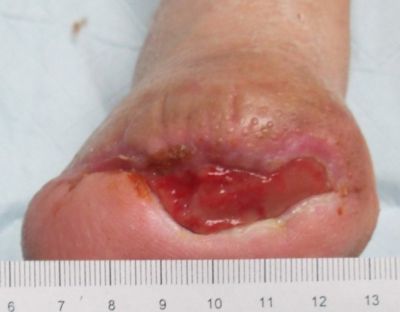
12-26-13
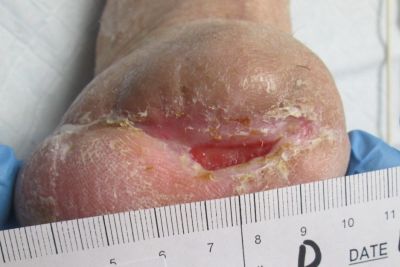
1-16-14
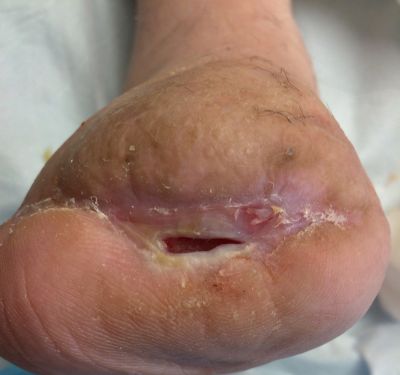
1-27-14
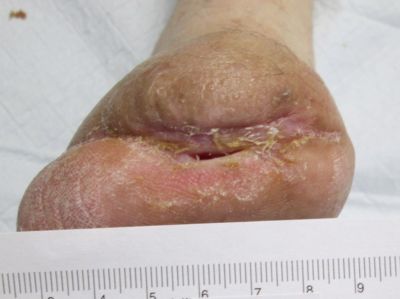
In the case of this patient, below knee amputation was the next step had the wound care team not offered the combination of epidermal autografting and NPWT. Surely this is only one of many case presentations you will read that resulted in the complete resolution of a seemingly unlikely to heal wound. But, the message is not simply about the technology used, or the treating provider and clinicians involved. What if we had been able to prevent or lessen the complexity of this case by assessing the patient's vascular status prior to her surgical procedure? In the field of wound care, developing technology to prevent wounds is becoming as much of a priority as the technology to heal.
The patient and the team were extremely satisfied that the novel* combination of two therapies resulted in successful limb salvage. Do you have a success story using combined therapies for a difficult to heal wound? Share your experience by commenting below! *I describe this as novel to our practice, but at the time of this case the KCI team had not been made aware of another which utilized these therapies in combination.
Referenced Products:
1. Mepilex® Transfer, Mölnlycke Health Care US, LLC
2. Mepitel® One, Mölnlycke Health Care US, LLC
3. CelluTome™ Epidermal Harvesting System, KCI - An Acelity Company
4. ActiV.A.C.® Therapy System, KCI - An Acelity Company
5. Arglaes® Powder, Medline Industries, Inc.
6. PROMOGRAN PRISMA® Matrix, Systagenix - An Acelity Company
7. Drawtex®, SteadMed Medical, LLC
8. PROMOGRAN® Matrix, Systagenix - An Acelity Company
9. Mepilex® Border, Mölnlycke Health Care US, LLC
Credit to treating providers/clinicians:
Dr. P. Shawn Hatfield, DPM, FACFAS and Samantha Kuplicki, BSN, RN, CWS, CWCN, CFCN
About the Author
Samantha Kuplicki is board certified in wound care by both the American Board of Wound Management as a Certified Wound Specialist (CWS) and by the Wound, Ostomy and Continence Certification Board as a Certified Wound Care Nurse (CWCN) and Certified Foot Care Nurse (CFCN). She serves on the American Board of Wound Management (ABWM) Examination Committee and also volunteers for the Association for the Advancement of Wound Care.
The views and opinions expressed in this content are solely those of the contributor, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.