Moisture-Associated Skin Damage (MASD)
Moisture-associated skin damage (MASD) is the general term for inflammation or skin erosion caused by prolonged exposure to a source of moisture such as urine, stool, sweat, wound drainage, saliva, or mucus. It is proposed that for MASD to occur, another complicating factor is required in addition to mere moisture exposure. Possibilities include mechanical factors (friction), chemical factors (irritants contained in the moisture source), or microbial factors (microorganisms). The moisture barrier of the skin plays a critical role in maintaining homeostasis within the body, mainly by concurrently slowing the movement of water out of the body (transepidermal water loss, or TEWL) and regulating the absorption of water and solutes from outside the body. When exposed to excessive amounts of moisture, the skin will soften, swell, and become wrinkled, all of which make the skin more susceptible to damage from one of the complicating factors mentioned above.
The four specific types of moisture-associated skin damage that will be discussed here are periwound moisture-associated dermatitis, peristomal moisture-associated dermatitis, incontinence-associated dermatitis, and intertriginous dermatitis.
Periwound Moisture-Associated Dermatitis
Etiology
The production of exudate is a normal result of the inflammatory stage of wound healing. However, the advent of moist wound healing has brought with it an understanding that moisture balance is the key to optimal outcomes. Excessive amounts of wound exudate can cause the periwound (within 4 cm of wound edge) skin to become macerated and even break down. This type of skin damage is call periwound moisture-associated dermatitis.
The chemical composition of the wound exudate greatly affects the potential damage that can be wrought. The presence of bacteria, specific proteins, or proteolytic enzymes, as well as the volume of wound exudate greatly reduce the barrier function of the skin and can lead to maceration. Specifically, exudate from chronic wounds has been found to contain a higher concentration of proteolytic enzymes as compared to exudate from acute wounds. Another factor affecting the occurrence of periwound maceration is damage to skin by aggressive removal of adhesive wound dressings, which affect the integrity of the skin barrier by stripping away parts of the epidermis.
Symptoms
Periwound moisture-associated dermatitis is marked by erythema (which may be harder to discern in persons with darkly pigmented skin), maceration (white, pale, or gray skin that is softened and/or wrinkled), and irregular or diffuse edges (as opposed to pressure ulcers which typically have distinct edges). Wounds with more viscous exudate are more prone to periwound maceration, as the moisture is less likely to be lost by evaporating through the dressing. The individual may report experiencing pain, burning or itching as a result of the skin damage. Damage may be focused on the dependent area of the wound in extremities, due to pooling of wound exudate.
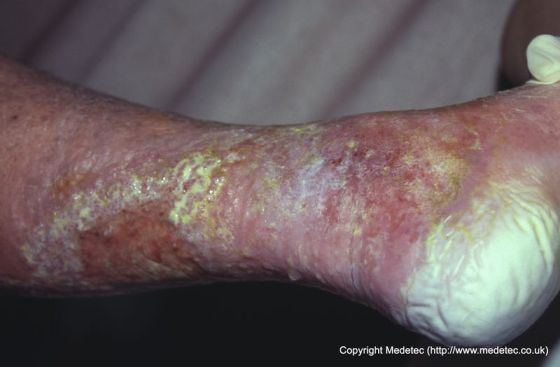 Figure 1: Example of periwound maceration
Figure 1: Example of periwound maceration
Risk Factors
The following wounds are more prone to developing periwound moisture-associated dermatitis:
- Diabetic foot ulcers
- Venous leg ulcers
- Pressure ulcers
- Fungating tumors
- Full-thickness (third-degree) burns
Wound infection will also greatly increase the risk of periwound maceration, as it increases the exudate production.
Treatment & Interventions
The following precautions can help minimize the risk of developing periwound moisture-associated dermatitis in at-risk patients and to minimize complications in patients already exhibiting symptoms:
- Monitor the wound area routinely for changes in skin condition.
- Manage wound exudate with dressings chosen for proper absorbency.
- Apply a barrier film or skin protectant to the periwound skin when appropriate.
The first step in treatment of periwound moisture-associated dermatitis is managing the excessive exudate. This can mean anything from absorptive or windowed dressings to external collection devices or negative pressure wound therapy in extreme cases. Liquid, ointment, or cream-based skin protectants offer a range of protection for the periwound skin from maceration. After exudate has been managed, the skin should be allowed to progress to healing.
Peristomal Moisture-Associated Dermatitis
Etiology
There are several types of moisture that can cause peristomal moisture-associated dermatitis, including exposure to urine or stool, sweat, wound drainage, or other sources of water such as while bathing or swimming. As part of the pouching process, solid skin barriers are placed around the stoma to protect the underlying skin from detrimental components of the stoma output (urine or stool). These barriers work to keep the skin dry by absorbing both effluent from the stoma and moisture from the underlying skin. If too much moisture is absorbed from the stoma, the barrier will cease to be effective, allowing the effluent to come in contact with the peristomal skin. Too much moisture underneath the barrier (sweat or exudate from an existing peristomal wound) can occlude the underlying skin and lead to maceration. Additionally, extended exposure of the pouch adhesive to water, typically while showering or swimming, can cause adhesive failure, requiring more frequent pouch changes and increasing the potential for mechanical damage from repeatedly removing the adhesive.
Symptoms
Leakage of stomal effluent onto the peristomal skin will cause inflammation and even skin erosion depending on the placement of the stoma (liquid and enzyme content varies along the length of the intestine). Maceration, which is marked by whitened and softened peristomal skin, is also common in cases where moisture is trapped under the skin barrier and the skin becomes occluded. The affected area may itch or be sore to the touch.
Risk Factors
The following factors increase the risk of developing peristomal moisture-associated dermatitis:
- Creasing of the skin underneath the skin barrier when changing positions (standing, sitting, supine)
- Degree of stomal protrusion
- Improper pouching technique and wear time
- Increased perspiration or exposure to external moisture (e.g. swimming, showering)
Treatment & Interventions
The following precautions can help minimize the risk of developing peristomal moisture-associated dermatitis in at-risk patients and to minimize complications in patients already exhibiting symptoms:
- Manage peristomal moisture sources such as perspiration, wound exudate, and external sources to ensure proper pouch adhesion.
- Make sure the pouch is not left in place for too long or too short of a period. Longer wear times may lead to compromised pouch adhesion and occlusion of the underlying skin, and shorter wear times can result in mechanical stripping of the skin
- When cutting or molding the skin barrier to fit the stoma, it is recommended that frequent measurements of the stoma be conducted over the first 6 weeks to adjust to the changing shape of the stoma.
Treatment of peristomal moisture-associated dermatitis will be geared towards preventing further irritation and healing the irritated skin. The pouching system should be reevaluated to ensure proper fitting and drainage, with the skin barrier suited to the type of output. Topical therapies such as skin barrier powders, pastes or rings can be used to absorb moisture under the skin barrier, provide an additional physical barrier, reduce existing irritation, and allow for proper adhesion of the solid skin barrier. If exudate from an underlying wound is the source of moisture, the etiology of the wound should be addressed and exudate managed with an appropriate absorptive dressing.
Incontinence-Associated Dermatitis
Etiology
Incontinence-associated dermatitis (IAD) is predominantly a chemical irritation resulting from urine or stool coming in contact with the skin. Ammonia from urine and enzymes from stool can disrupt the acid mantle of the skin and eventually cause the skin to break down. As with the other forms of MASD discussed above, maceration also plays a key role in the formation of IAD, and can makes the skin more susceptible to friction damage. While urinary incontinence may lead to IAD, it is much more common in individuals with fecal incontinence or mixed urinary and fecal incontinence.
The affected area will present with erythema, as well as maceration. The area may progress to painful partial-thickness erosions with weepy serous exudate. If left untreated, pressure and friction may increase stress on the affected area, leading to skin breakdown. Depending on the areas exposed to urine and stool, IAD is not necessarily limited to the perineal area, and can extend up onto the lower back or down onto the inner thighs.
Risk Factors
- Use of containment or absorbent products, which can lead to excessive occlusion and maceration
- Fecal or mixed urinary/fecal incontinence
- Toileting ability
Treatment & Interventions
The following precautions can help minimize the risk of developing incontinence-associated dermatitis in at-risk patients and to minimize complications in patients already exhibiting symptoms:
- Minimize skin exposure to urine and stool.
- Develop a consistent regimen of skin care to protect the integrity of the skin barrier, including cleansing, moisturizing and use of a skin protectant.
If measures can be taken to address the corresponding incontinence, these should be considered while steps are taken to implement a skin care regimen to protect the skin from continued irritation. After the skin has been properly cleansed and moisturized, a skin barrier should be applied to protect the affected skin from further exposure. Any secondary infection of the affected area should be treated topically. In some cases, a containment or diversion device may be indicated.
Intertriginous Dermatitis
Etiology
Intertriginous dermatitis (ITD), also referred to as intertrigo, results from sweat being trapped in skin folds with minimal air circulation. When the sweat is not able to evaporate, the stratum corneum becomes overly hydrated and macerated, facilitating friction damage that is often mirrored on both sides of the fold. This in turn leads to inflammation and denudation of the skin, making the area more prone to infection. ITD typically affects infants because of their exaggerated skin folds and stooped posture, obese individuals, and, in the case of ITD affecting the webbing between the toes, active individuals that wear closed toe or tight-fitting shoes.
In addition to having more skin folds, obese individuals also present with several physiological factors that can increase the risk of developing ITD. These include an increase in perspiration to regulate body temperature, increased transepidermal water loss (TEWL), and higher skin surface pH (which makes the acid mantle less effective as a natural barrier to infection).
Symptoms
ITD typically presents with mild erythema that may progress to more severe inflammation, erosion, oozing, exudation, maceration, and crusting of the intertriginous skin mirrored on both sides of the fold. The individual may report pain, itching, or burning sensations around the affected area. With toe web ITD, the webbing may present with maceration, erythema, desquamation and even erosion of the affected skin, impairing ambulation in severe cases.
Risk Factors
- Obesity
- Diabetes mellitus
- Urinary and fecal incontinence
- Hyperhidrosis
- Poor hygiene
- Malnutrition
- Drooling (in infants)
- Individuals who are bedridden
Treatment & Interventions
The following precautions can help minimize the risk of developing intertriginous dermatitis in at-risk patients and to minimize complications in patients already exhibiting symptoms:
- Reduce heat and moisture within the skin fold
- Keep at-risk areas clean and dry
- Shower after exercise, then thoroughly pat dry the skin inside the fold
- Use a pH-balanced skin cleanser
- Promote proper general skin hygiene
The goal of treatment for intertriginous dermatitis is to minimize moisture and friction in the skin fold and to treat any infections. Topical or oral treatments should be used for any secondary fungal or bacterial infections. If weight loss is possible, this will reduce future complications. While not often recommended specifically as treatment for ITD, surgical removal of redundant skin will also serve to reduce the risk of developing ITD. Light, non-constricting, absorbent clothing made of natural fibers is recommended to promote air circulation and moisture vapor transmission. Silver wicking textiles or absorptive dressings may be placed in the skin fold to inhibit microbial growth and absorb moisture. For toe web ITD, open-toed shoes are recommended to promote air circulation.
Image Credit: Medetec (http://medetec.co.uk/). Used with permission.
References
Alvey B, Beck DE. Peristomal Dermatology. Clin Colon Rectal Surg. 2008;21(1):41-44. doi: 10.1055/s-2008-1055320
Black JM, Gray M, Bliss DZ, Kennedy-Evans KL, Logan S, Baharestani MM, Colwell JC, Goldberg M, Ratliff CR. MASD Part 2: Incontinence-Associated Dermatitis and Intertriginous Dermatitis. J Wound Ostomy Continence Nurs. 2011;38(4):359-370. doi: 10.1097/WON.0b013e31822272d9
Colwell JC, Ratliff CR, Goldberg M, Baharestani MM, Bliss DZ, Gray M, Kennedy-Evans KL, Logan S, Black JM. MASD Part 3: Peristomal Moisture–Associated Dermatitis and Periwound Moisture–Associated Dermatitis. J Wound Ostomy Continence Nurs. 2011;38(5):541-553. doi: 10.1097/WON.0b013e31822acd95
Gray M. Incontinence-Related Skin Damage: Essential Knowledge. Ostomy Wound Manage. 2007;53(12):28-32.
Gray M, Black JM, Baharestani MM, Bliss DZ, Colwell JC, Goldberg M, Kennedy-Evans KL, Logan S, Ratliff CR. Moisture-Associated Skin Damage: Overview and Pathophysiology. J Wound Ostomy Continence Nurs. 2011;38(3):233-241. doi: 10.1097/WON.0b013e318215f798
Janniger CK, Schwartz RA, Szepietowksi JC, Reich A. Intertrigo and Common Secondary Skin Infections. Am Fam Physician. 2005;72(5):833-838. http://www.aafp.org/afp/2005/0901/p833.html. Published September 1, 2005. Accessed January 24, 2019.
Ousey K, Bianchi J, Beldon P, Young T. The Identification and Management of Moisture Lesions. Wounds UK. 2012;8(2):S1-20. https://www.wounds-uk.com/journals/issue/30/article-details/supplement-8... Accessed January 24, 2019.
Thomas S. The role of dressings in the treatment of moisture-related skin damage. World Wide Wounds. http://www.worldwidewounds.com/2008/march/Thomas/Maceration-and-the-role.... Published March 2008. Accessed January 24, 2019.
Zulkowski K. Diagnosing and Treating Moisture-Associated Skin Damage. Adv Skin Wound Care. 2012;25(5):231-236. doi: 10.1097/01.ASW.0000414707.33267.92.







