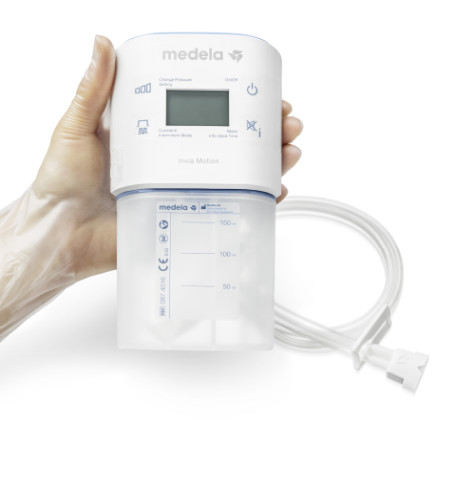
Invia Motion® Negative Pressure Wound Therapy System
Invia Motion® Negative Pressure Wound Therapy System is for treating a variety of NPWT wounds in the acute, extended and, home care settings. Available in 3 different settings (15 day, 60 day, and Endure 3 years).
Medela, LLC
Medela provides an innovative portfolio of products and services for negative pressure wound therapy that includes devices, gauze and foam dressing kits, Invia® Silverlon® dressings, and accessories.Benefits
Invia® NPWT Systems contain a double lumen with Intelligent Pressure Control™ and Dynamic Exudate™ technology
• Intelligent Pressure Control technology ensures the set pressure is delivered at the wound bed
• Dynamic Exudate Removal technology actively prevents tubing blockages
• Provides NPWT across the continuum of care - from hospital to home
• Clear user interface supported by four buttons and a digital display
• Discreet carrying case, battery autonomy of 10 hours, and compact design for comfort and mobility
• Preset pressure settings from -40mmHg to -175mmHg (-125mmHg default setting)
• Easy and secure tubing attachment with the quick-connector
• Choice of therapy run times to meet clinical needs (15-day, 60-day, Endure 3 years)
• Delivers the convenience of a personal pump while offering clinical flexibility with a choice of pressure settings, therapy modes, and compatibility with both foam and gauze dressings
• Appropriate for use in all patient care settings
• Small blockages in the tubing are resolved by the pump
• Clinicians and patients are notified of any unresolved blockages
Indications
The Invia Motion® NPWT system is appropriate for the following indications: acute or subacute wounds, chronic wounds, dehisced wounds, pressure injuries, diabetic/neuropathic ulcers, venous insufficiency ulcers, traumatic wounds, partial-thickness burns, flaps and grafts, and closed surgical incisions.
Contraindications
The Invia Motion® NPWT system is contraindicated in the presence of: necrotic tissue with eschar present, untreated osteomyelitis, non-enteric and unexplored fistulas, malignancy in the wound, exposed vasculature, exposed nerves, exposed anastomotic site of blood vessels or bypasses, or exposed organs.
Warnings and Precautions
Refer to Instructions for Use (IFU).
Storage Requirements
Store Starter Kit between 15% and 93% humidity and -20° to 50°C (-4° to 122°F).
How Supplied/Sizing
Product features
Other features
Recommended Use
Acute Wounds
Burns
Cavity Wounds
Chronic Wounds
Deep Wounds
Dehisced Wounds
Diabetic Foot
Diabetic/Neuropathic Ulcers
Flaps and Grafts
Granulating/Epithelializing Wounds
Moderate/Highly Exudating Wounds
Open Wounds
Palliative Wounds
Partial-Thickness Burns
Pressure Ulcers
Surgical Wounds
Traumatic Wounds
Venous Ulcers
Mode of Use/Application
Refer to Instructions for Use (IFU).
Removal & Change Frequency
Routine dressing changes should occur every 48-72 hours. Consider more frequent dressing changes for infected wounds.
Dressing (wound contact layer, wound filler, wound cover, drain, and external suction interface (ESI)) should be changed every 48 to 72 hours, but no less than 3 times a week, or as instructed by the health care professional.
Change canister/tubing set when full or with dressing changes.
Additional Recommended Dressings
For use with both Invia® Gauze Dressing Kits, Invia® Foam Dressing Kits, and Invia® Silverlon®.
Construction
Consists of metal, plastic, electrical, and battery components.
Warranty
Refer to Instructions for Use (IFU).
Clinically Tested
Latex-friendly
Non-allergenic
Non-comedogenic
Non-cytotoxic
Non-irritating
Technical Specifications
Vacuum range: -60mmHg, -80mmHg, -100mmHg, -125mmHg, -150mmHg, -175mmHg
Flow: 1 L/min
Weight: 0.88lb or 700g without canister
IP33
H x W x D: 3.9"x3.8"x2.05"
ISO 9001, 13485
CE (93/42/EEC, IIa)
Max noise level: 46 db(A)
Operation of device: between 15% and 93% humidity, 5°C to 40°C, and 70kPa-106kPa atmospheric pressure
Store/transport device: between 15% and 93% humidity, -20°C-50°C, and 70kPa-106kPa atmospheric pressure
Battery Type: NiMH
Battery Life During Routine Use: approximately 10 hours
Battery Recharge Time: 4 hours
Tubing: silicone (medical grade)
Patient Cone Connector: polypropylene
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More










