Slough: What Is This Stuff?
January 20, 2023

Editor's note: This blog by Dianne Rudolph, DNP, APRN, GNP-BC, CWOCN, is the recipient of the 2023 Blog of the Year Award. It has received the most views of any blog posted on WoundSource within the last year. The WoundSource Editors would like to congratulate Dr. Rudolph on her award.
Introduction: Slough Versus Eschar
Nonviable tissue in the wound bed can be divided into 2 broad categories: slough and eschar. Although these terms are sometimes used interchangeably, it is vital to distinguish between them as they may require different management methods.  Dry, hard, leathery tissue in the wound bed is referred to as Eschar (Figure 1). Eschar is a type of necrotic tissue that is secondary to cell death following tissue injury (ie, pressure, trauma, impaired perfusion).
Dry, hard, leathery tissue in the wound bed is referred to as Eschar (Figure 1). Eschar is a type of necrotic tissue that is secondary to cell death following tissue injury (ie, pressure, trauma, impaired perfusion).
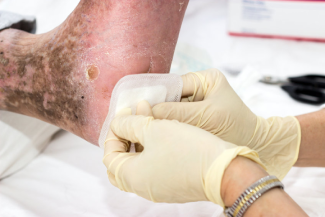
Slough, in comparison, is usually seen as well hydrated, soft yellow or white tissue. (Figure 2) This tissue may be loose and stringy or adherent and is the byproduct of the inflammatory phase of wound healing.1-2  When wounds fail to proceed through the normal phases of wound healing (hemostasis, inflammation, proliferation, and maturation/remodeling), they can become chronic. Most chronic wounds are a result of prolonged inflammation. Common features of non-healing wounds include the following:
When wounds fail to proceed through the normal phases of wound healing (hemostasis, inflammation, proliferation, and maturation/remodeling), they can become chronic. Most chronic wounds are a result of prolonged inflammation. Common features of non-healing wounds include the following:
- Heavy exudate
- Recurrent infection
- Tissue necrosis
- Impaired epithelization
- Decreased angiogenesis
- Overproduction of reactive oxygen species
Slough, as a form of necrotic tissue, contains ingredients such as fibrin, leukocytes, dead cells, microbes, and proteinaceous materials.2-4 Slough is different from biofilm. Biofilm is usually seen as a slimy, gelatinous substance on the surface of a wound. It is recognized that bacteria exist naturally not as free-floating individuals and planktonic micro-organisms but as sessile communities attached to a surface. Where these communities form permanent attachments and produce an extracellular polysaccharide (EPS) matrix, they may be described as biofilms. The human body has many surfaces on which these can occur, including acute and chronic wounds. It is also possible for biofilms and slough to coexist in a chronic wound.5
Why is Slough Such a Problem in Chronic Wounds?
The presence of slough in the wound bed is a deterrent to healing. The appropriate and safe removal of slough is a vital component of wound healing. Slough makes it difficult for clinicians to assess the wound bed accurately and contributes to delayed wound healing. The presence of slough can prolong the inflammatory phase of healing, provides a nidus for infection by attracting bacteria to the wound bed, increases odor and exudate, and may prevent the wound from continuing through the normal wound healing process.1-3
De-sloughing or debriding a wound is critical to provide appropriate, evidence-based wound management. It is imperative, however, to distinguish between slough and necrotic tissue. The goal of care with slough is to remove it. Necrotic tissue, such as eschar, should not be removed in the presence of untreated arterial disease, gangrene, fungating or ulcerating tumors, and wounds with an underlying inflammatory process such as pyoderma gangrenosum. Stable eschar on heels should be left intact unless there is adequate tissue perfusion.1-2
What Clinicians Should Consider When Removing Slough
Prior to debridement, the procedure must be fully explained to the patient, and consent should be gained. The method chosen will depend on a number of factors, including the following, although not limited to1,6-7:
- The wound’s underlying pathology
- Any comorbidities
- The potential risk of bleeding
- The extent of slough tissue in the wound bed (small versus large area)
- The patient’s level of pain
- Availability of resources
- Skill level of the wound care professional
- The procedure’s location (inpatient, outpatient, or home community setting)
Methods for debridement include autolytic, enzymatic, biosurgical, surgical, mechanical, and ultrasonic.
Autolytic Debridement
Autolytic debridement is the lysis, or breakdown, of damaged tissue at a wound site by the body's natural defense system through enzymes that digest specific components of body tissues or cells, like proteins, fibrin, and collagen. It is thought that this process can be encouraged using dressings such as hydrocolloids, hydrogels, alginates, and transparent films that support moisture maintenance and provide optimal conditions for the body's natural enzymes to activate wound debridement. This process is typically slower than most but is well tolerated and appropriate for most patients.7-8
Enzymatic Debridement
Enzymatic debridement is a selective method for the debridement of necrotic tissue using an exogenous proteolytic enzyme, collagenase, to debride the tissue. Collagenase digests the collagen in the necrotic tissue, allowing it to detach. Enzymatic debridement is a slower method of debridement in comparison to mechanical and sharp debridement. Collagenase and moisture-retentive dressings can work synergistically, enhancing the debridement. Enzymatic debridement is not recommended for patients with known sensitivity to the product's ingredients.9
Biological Debridement
Biosurgical or Maggot debridement therapy introduces live, sterile fly larvae into a wound to facilitate selective debridement and healing. This technique was discovered as a beneficial effect of the colonization of human tissue by fly larvae, called myiasis. This discovery was made during World War I when it was observed that injured soldiers whose wounds were infested with maggots healed faster than their counterparts whose wounds were free from maggots.10-11 In this therapy, the larvae of Blow fly (Lucilia sericata) are used because they feed exclusively on dead tissues.
There are 2 ways these maggots are applied into the wound, namely, free range and biobag dressing. The mechanism of action of the maggots during debridement involves the secretion of digestive enzymes, which breakdown the dead tissues, liquefying them so that the maggots can easily ingest them. The maggots’ secretions also inhibit microbial growth in the wound, thereby mitigating infection risk. This therapy can successfully treat leg ulcers, pressure injuries, and diabetic wounds. The advantages of maggot debridement therapy are enormous. The cost is relatively low, it quickens wound healing, and it is painless. However, the patient may experience irritation and itching at the wound site, which is associated with larval movement in the wound. Some wound care professionals have reservations about this treatment’s use due to the nature of their activity and that the treatment includes live insects.10-11
Surgical Debridement
Surgical debridement, or conservative sharp debridement, is performed with a scalpel, curette, or scissors to excise necrotic tissue. Surgical debridement is indicated for the removal of both slough and eschar. Tissue is frequently so that a margin of normal tissue is exposed if possible. This tissue exposure is usually accomplished in the operating room when extensive debridement is required, whereas conservative debridement can be done at the bedside in most settings.7 Figures 3a and 3b depict before and after images of a wound undergoing conservative sharp debridement.


Mechanical Debridement
Mechanical debridement involves the physical removal of necrotic debris from a wound. A wide range of methods are used in clinical practice that include the following:
- Wet-to-dry dressing changes
- Hydrotherapy
- Monofilament pads
- Wound irrigation
Wet-to-dry dressings involve the application of moist gauze to a wound bed. After a specified period, the dressing will dry out, which allows the tissue to adhere to the gauze. When the dressing is changed, the necrotic tissue and slough that adhered to the gauze are also removed. This type of debridement is also referred to as non-selective debridement, as both healthy and unhealthy tissue get removed in this process. This form of debridement may be a painful process for many patients. Hydrotherapy refers to the use of water to remove dead and other types of unwanted tissues. There are various devices on the market for this treatment that can be very effective. Monofilament pads are made of 100% unbleached monofilament fibers and a polyacrylate coating. They can safely clean wounds by removing slough, debris, biofilm, and bacterial load. This debridement allows for virtually painless cleansing of superficial and chronic wounds, including diabetic and pressure ulcers and third-degree burns.11-12
Ultrasonic-assisted Wound Debridement
Ultrasonic-assisted wound (UAW) debridement is a method that uses low-frequency ultrasound waves. This tool allows for precise surgical debridement layer by layer, from superficial to deep, while protecting underlying viable tissues. Variations include contact and non-contact ultrasound. The use of contact ultrasound appears to be somewhat more efficacious in wound debridement. Three clinical effects of ultrasound include selective tissue debridement, wound stimulatory effects, and antibacterial activity that may facilitate the early healing of wounds. These clinical effects that promote wound healing, in turn, can reduce the cost to the health care system and improve the patient's quality of life.11,13-14 Figure 4a and 4b depict before and after images of a wound undergoing treatment with low frequency contact ultrasound.


Conclusion
Slough is a specific type of nonviable tissue that occurs as a byproduct of the inflammatory process. It is more common in chronic wounds and presents as a yellowish, moist, stringy substance. It can delay healing and increase the risk of infection. Slough removal is a critical part of wound management and can be accomplished by any number of debridement methods, as outlined above. Debridement methods can also be combined for enhanced results. For example, enzymatic debridement with serial sharp debridements can be very effective. Specific choices will depend upon clinician skill level, availability of techniques, and tolerance by the patient.
Resources
- Angel D. Slough: what does it mean and how can it be managed. Wound pract res. 2019;27(4). doi:10.33235/wpr.27.4.164-167
- McGuire J, Nasser J. Redefining slough: A new classification system to improve wound bed assessment and management. Wounds. 2021;33(8):E61-E66. doi:10.25270/wnds/2021.e6166
- Alam W, Hasson J, Reed M. Clinical approach to chronic wound management in older adults. J Am Geriatr Soc. 2021;69(8):2327-2334. doi:10.1111/jgs.17177
- Raziyeva K, Kim Y, Zharkinbekov Z, et al. Immunology of acute and chronic wound healing. Biomolecules. 2021;11(5):700. doi:10.3390/biom11050700
- Thomson CH. Biofilms: do they affect wound healing? Int Wound J. 2011;8(1):63-67. doi:10.1111/j.1742-481X.2010.00749.x
- Thomas DC, Tsu CL, Nain RA, et al. The role of debridement in wound bed preparation in chronic wound: A narrative review. Ann Med Surg (Lond). 2021;71(102876):102876. doi:10.1016/j.amsu.2021.102876
- Salati SA. Debridement – a review of current techniques. J Pak Assoc Dermatol. 2021;31(2):262-272. Accessed December 26, 2022. http://www.jpad.com.pk/index.php/jpad/article/view/1604
- Choo J, Nixon J, Nelson A, et al. Autolytic debridement for pressure ulcers. Cochrane Libr. Published online 2019; doi:10.1002/14651858.cd011331.pub2
- Manna B, Nahirniak P, Morrison CA. Wound Debridement. StatPearls [Internet]. 2022; https://www.ncbi.nlm.nih.gov/books/NBK507882/
- Nnachi IA, Okeanya BC, Ezinwa HC. Maggot debridement therapy and innovation from myiasis - A review. Bio-Res. 2022;20(3):1721-1729. doi:10.4314/br.v20i3.9
- Pajarillo C, Sherman RA, Sheridan R, et al. Health professionals’ perceptions of maggot debridement therapy. J Wound Care.2021;30(Sup9a):VIIi-VIIxi. doi:10.12968/jowc.2021.30.Sup9a.VII
- Stiehl JB. Early wound bed preparation: irrigation and debridement. J Wound Care. 2021;30(Sup9):S8-S16. doi:10.12968/jowc.2021.30.Sup9.S8
- Kataoka Y, Kunimitsu M, Nakagami G, et al. Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: A scoping review. Int Wound J. 2021;18(2):176-186. doi:10.1111/iwj.13509
- Chen H, Yu Z, Liu N, et al. The efficacy of low-frequency ultrasound as an added treatment for chronic wounds: A meta-analysis. Int Wound J. 2022; doi:10.1111/iwj.13893
About the Author
Dr. Dianne Rudolph, DNP, APRN, GNP-BC, CWOCN is a nurse practitioner board-certified in Gerontological advanced practice nursing and as a wound, ostomy and continence nurse. Dr. Rudolph is currently working as an independent consultant in wound care. She has been a nurse for more than 30 years with experience in trauma care, acute care, home care, hospice, long term care, rehab, and wound care. She is very passionate about caring for adults and older adults with complex medical and wound needs. She has been a faculty member for several schools of nursing and is currently adjunct faculty at the University of Texas Health Science Center in Houston. She has presented multiple lectures and has published articles and book chapters on a variety of topics.
The views and opinions expressed in this content are solely those of the contributor, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.